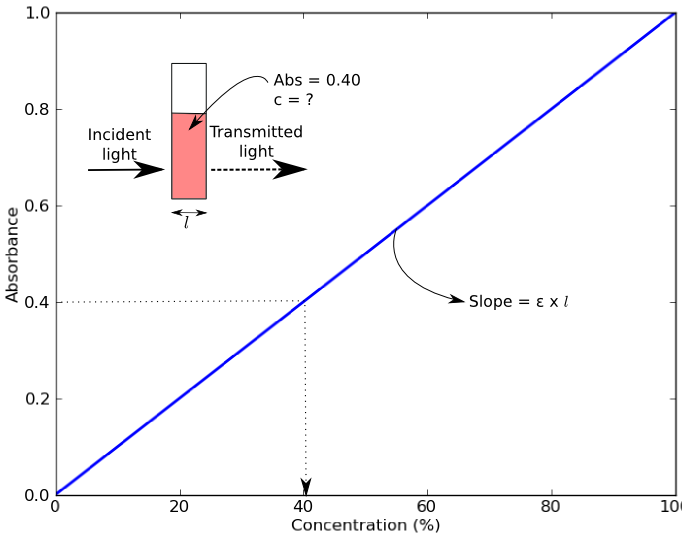
Beer's Law What Is E . Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution absorbs. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
from stuff.iorodeo.com
Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. In other words, a solution absorbs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User
Beer's Law What Is E Beer’s law suggests that a plot of absorbance vs. Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. Beer’s law suggests that a plot of absorbance vs. In other words, a solution absorbs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer's Law What Is E Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that the concentration of a chemical solution is directly proportional to its. Beer's Law What Is E.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer's Law What Is E In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation. Beer's Law What Is E.
From www.youtube.com
B.7 The Beer Lambert law (HL) YouTube Beer's Law What Is E In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law suggests that a plot of absorbance vs. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that the concentration. Beer's Law What Is E.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law What Is E In other words, a solution absorbs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law suggests that a plot of absorbance vs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that. Beer's Law What Is E.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law What Is E Beer’s law suggests that a plot of absorbance vs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly. Beer's Law What Is E.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law What Is E In other words, a solution absorbs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law suggests that a plot of absorbance vs. Beer’s law, in spectroscopy, a. Beer's Law What Is E.
From studylib.net
Beer Lambert Law Beer's Law What Is E Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law suggests that a plot of absorbance vs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In spectroscopy, beer’s law states that the absorption of light by a sample is directly. Beer's Law What Is E.
From www.teachertube.com
AP Beer's Law Problems 15 Beer's Law What Is E Beer’s law suggests that a plot of absorbance vs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In other words, a solution absorbs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law states that the concentration of a chemical. Beer's Law What Is E.
From www.slideserve.com
PPT Review of Basic Concepts, Molarity, Solutions, Dilutions and Beer Beer's Law What Is E Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. In spectroscopy, beer’s. Beer's Law What Is E.
From www.slideserve.com
PPT Exp 14B Determining an Equilibrium Constant PowerPoint Beer's Law What Is E Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In other words, a solution absorbs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an. Beer's Law What Is E.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law What Is E Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law suggests. Beer's Law What Is E.
From www.slideserve.com
PPT Instrumental analysis PowerPoint Presentation, free download ID Beer's Law What Is E In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing. Beer's Law What Is E.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law What Is E Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. Beer’s law suggests that a plot of absorbance vs. In other words, a solution absorbs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer's law states that the. Beer's Law What Is E.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer's Law What Is E Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. Beer’s law suggests that a plot of absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Beer lamberts law states a relationship between the attenuation of light through a substance. Beer's Law What Is E.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law What Is E Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer lamberts law states a relationship between the attenuation of. Beer's Law What Is E.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 Beer's Law What Is E Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance,. Beer's Law What Is E.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law What Is E In other words, a solution absorbs. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer’s law suggests that a plot of absorbance vs. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer's law states that. Beer's Law What Is E.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer's Law What Is E Beer's law states that the concentration of a chemical solution is directly proportional to its absorption of. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. In spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. Beer’s law. Beer's Law What Is E.